Recently, new energy vehicles that "can run with water" are well known. Although there are many doubts about this project invested by the local government, the production of hydrogen from water is not a myth, but it has been studied by scientists. The new technology, its preparation statement is electrochemical hydrogen evolution (HER), is an important way to obtain high-purity hydrogen and achieve sustainable distributed storage.
The important factor for HER to produce hydrogen is the catalyst. How to design and prepare an efficient catalyst to drive the HER reaction is the main challenge to promote the practical application of this method. In response to the disadvantage of commercial Pt / C catalysts that are costly and difficult to scale up, researchers have developed many low-cost transition metal compounds (such as sulfides, phosphides, and carbides) as alternative catalysts and have achieved some results.
In addition, with the development of single atom catalyst preparation and characterization technology in recent years, from the perspective of reducing metal loading and maximizing atom utilization, it has become possible to prepare catalysts using Pt designs with high intrinsic activity.
The University of Science and Technology of China announced that Professor Song Li from the National Synchrotron Radiation Laboratory of the University of Science and Technology of China and Professor Jiang Jun from the Hefei National Research Center for Microscale Material Science have collaborated on the design of low platinum (Pt) supported catalysts and their electrochemical hydrogen evolution performance Significant progress has been made in this area, revealing the effect of local electric field effects on the kinetic process of the HER reaction. Related achievements were published in the internationally renowned magazine Nature Energy under the title of "Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution".
The active sites of single-atom catalyst metals are usually evenly distributed on the support. Under the condition of uniform active site structure, from the perspective of chemical reaction collision theory, the strategies to further improve the performance of the catalyst are: (1) preparation of high-density monoatomic catalyst (but need to increase the amount of metal); (2) increase the reactants Local concentration at the active site of the catalyst. In particular, the tip structure of the catalyst can have a local electric field, which acts on the chemical reaction, and the reaction substrate will have a local high concentration distribution around the tip structure of the catalyst, so that it is expected to accelerate the kinetic process of the chemical reaction, and ultimately obtain a better Catalytic performance.
Based on this, the research group of Professor Song Li of the University of Science and Technology of China, based on the preliminary work of nano-diamonds, and Professor Jiang Jun and other collaborators through precise synthesis combined with theoretical design, successfully obtained a high curvature quasi-0D carbon nano onion (OLC) loaded Pt sheet Atomic catalyst. The test results show that the Pt1 / OLC catalyst with lower Pt loading (0.27 wt%) in acidic medium exhibits low overpotential (38 mV at 10 mA cm-2) and high TOF value (40.78 H2 s-1at 100 mV ), Not only superior to the Pt1 / graphene catalyst with similar Pt loading (0.33 wt%) prepared by the same method, but also close to the performance of the commercial 20 wt% Pt / C catalyst. Based on the first-principles method, theoretical calculations indicate that the hydrogen production activity of the Pt site is high. Synchrotron radiation X-ray spectroscopy combined with high-resolution electron microscopy observations found that thanks to the high-curvature OLC carrier surface, the Pt site constitutes a tip and produces a local electric field effect, which induces protons to gather around the Pt site and promotes proton coupling The electron transfer (PECT) process finally showed excellent HER performance. This work proposes a new strategy to enhance the activity of single-atom sites by regulating the structure of nano-carbon carriers, and also provides an effective characterization approach based on the fine structure analysis and theoretical calculation of synchrotron radiation.
The corresponding authors are Professor Song Li (http://staff.ustc.edu.cn/~song2012/) and Professor Jiang Jun (http://staff.ustc.edu.cn/~jiangju1/), co-first authors For Dr. Liu Daobin and Dr. Li Xiyu. The work began with the purification of nanodiamonds in 2012 and was supported by projects such as the National Major Scientific Research Program (MOST) and the National Natural Science Foundation of China (NSFC) of the Ministry of Science and Technology. At the same time, Shanghai Light Source, Beijing Synchrotron Radiation Device, Hefei National Synchrotron Radiation The laboratory and the Micro and Nano Research and Manufacturing Center of the University of Science and Technology of China provided experimental conditions for the development of the subject.
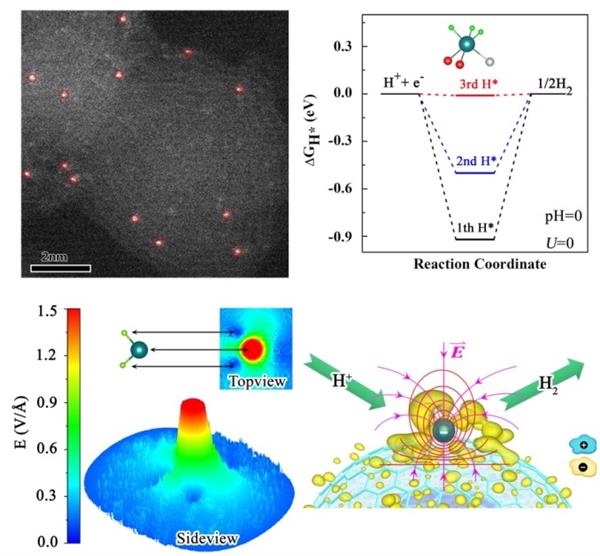
Design of Pt Monoatomic Catalyst Supported on Carbon Nano Onion with High Curvature and Its Mechanism of Hydrogen Evolution (HER)
Pvc Mooulding Capping,Building Moulding Capping,Plastic Moulding Capping,4.5Mm Building Moulding Capping
TAISHAN CITY WANAN CABLE CO.,LTD , https://www.tswanan.com