Under the guidance of the national subsidy policy, the ternary material system with higher energy density has completely replaced lithium iron phosphate batteries in the field of passenger cars, and with the continuous improvement of the energy density of power batteries in recent years, ternary materials have also gradually The transition from NCM111 to NCM532 and NCM622 systems, and is accelerating the transition to NCM811 system, we know that for ternary materials, there is a close relationship between the Ni content and its reversible capacity, the higher the Ni content, the higher the reversible capacity of the material For example, some scholars have tested NCM materials with Ni content as high as 0.9. The reversible capacity is more than 220mAh / g, but the higher the Ni content, the worse the stability of the ternary material and the worse the cycle life. Therefore, the ternary material passes The method of increasing the Ni content to increase the reversible capacity has also come to an end.
Recently, Chen Liquan, Huang Xuejie, and Xiaohui Rong (first author) of the Beijing Institute of Physics, Chinese Academy of Sciences and others have developed a P2 type Na0.72 [Li0.24Mn0.76] O2 sodium ion battery cathode material through an anion redox reaction mechanism. Its highest reversible capacity can reach 270mAh / g, achieving O2-high stable redox for the first time, providing a possible choice for the development of next-generation high-capacity cathode materials.
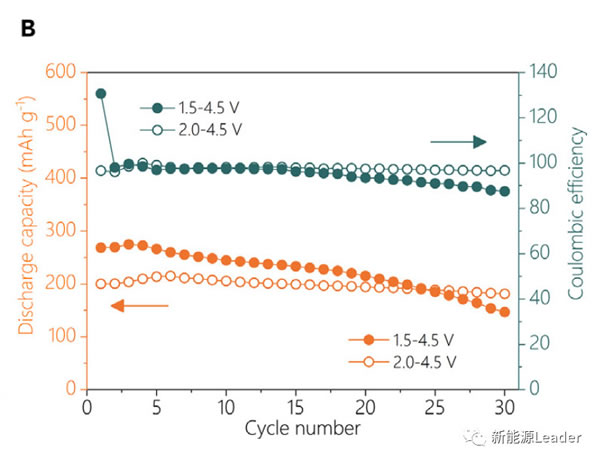
In the experiment, Xiaohui Rong synthesized Na0.72 [Li0.24Mn0.76] O2 material by solid-phase reaction method. From the figure a below, we can see that the reversible capacity of the material can reach 270mAh / g between 1.5-4.5V. It is currently the anode material of sodium ion battery with the highest reversible capacity. From figure b below, you can see that Na0.72 [Li0.24Mn0.76] O2 material can get very good cycle performance if it is cycled between 2.0-4.5V.
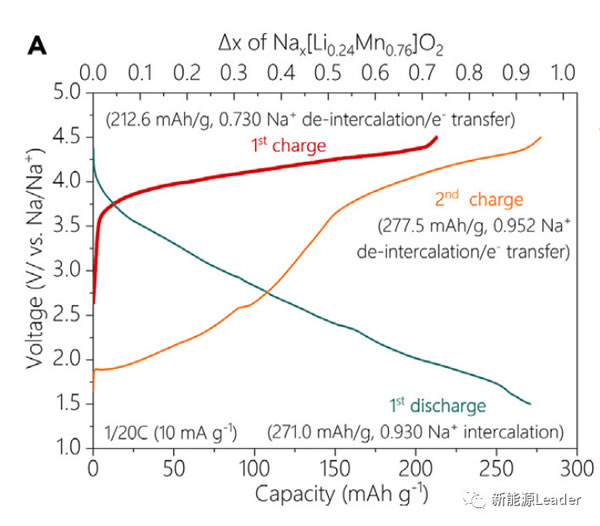
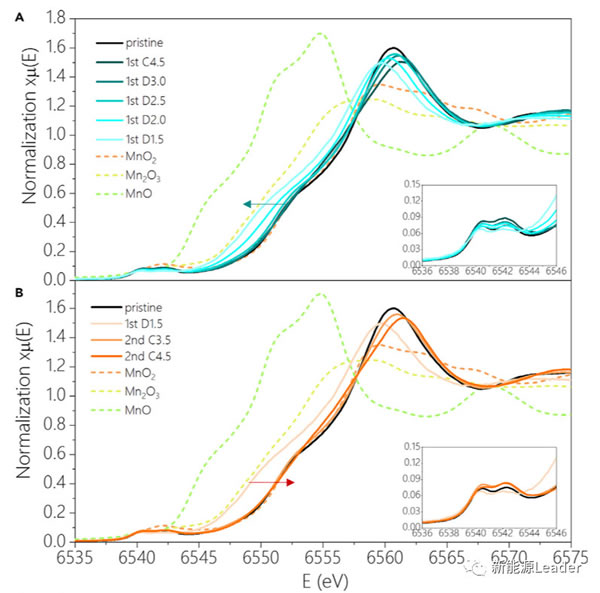
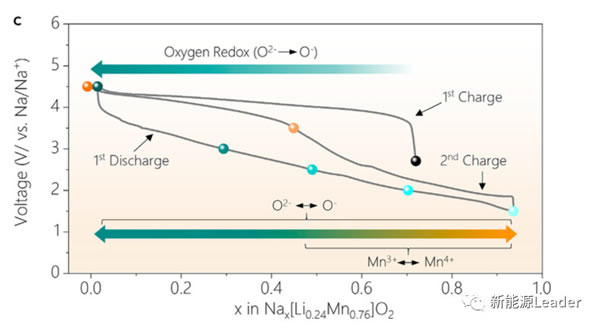
The high capacity of 270mAh / g has far exceeded the capacity provided by the Mn3 + / Mn4 + redox pair, so where do the electrons needed for the additional capacity come from? In order to answer this question, Xiaohui Rong analyzed the K-edge X-ray absorption spectrum of Mn element under different charging cut-off voltage conditions (as shown in the figure below). From the figure below, we can see the first charge In the process, we see that the K-edge absorption structure of Mn element has not changed significantly, but only a slight deviation has occurred, which also shows that the valence state of Mn element has not changed basically during the first charge, so the first charge The capacity mainly comes from the oxidation reaction of O element. During the discharge process, the K-side absorption structure of the Mn element has not changed significantly in the range of 4.5-2.5V, which indicates that the capacity provided in this voltage range is mainly the reduction reaction of the O element. If the discharge continues At 1.5V, it can be observed that the K-side absorption structure of Mn element will shift towards the low energy region, which indicates that the valence state of Mn element has decreased, which indicates that the capacity in the lower voltage range mainly comes from Mn3 + / Mn4 + oxidation The reaction of the reduction pair, and the capacity in the higher voltage range mainly comes from the redox reaction of the element O.
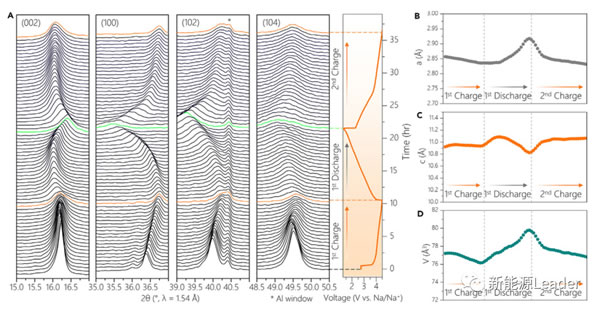
The Mn-based sodium ion battery cathode material is often accompanied by the phase change of the material itself during the Na + extraction and insertion process (for example, from the P2 phase to the O2 phase), causing a series of problems such as serious volume changes, which seriously affects the sodium ion battery Cyclic stability of cathode materials. Therefore, Xiaohui Rong also used the in-situ XRD method to analyze the structural changes in the process of Na0.72 [Li0.24Mn0.76] O2 material insertion and extraction of Na + (the results are shown in the figure below). It can be seen that only the characteristic peaks shifted during the entire charge and discharge process, for example, (100), (102) and (104) several characteristic peaks shifted to a higher angle during the charging process , But did not form a new characteristic peak, which also shows that in the charge and discharge process Na0.72 [Li0.24Mn0.76] O2 material did not form a new O2 or OP4 phase, indicating Na0.72 [Li0.24Mn0. 76] O2 materials have good structural stability, which is also a prerequisite for good cycle performance.
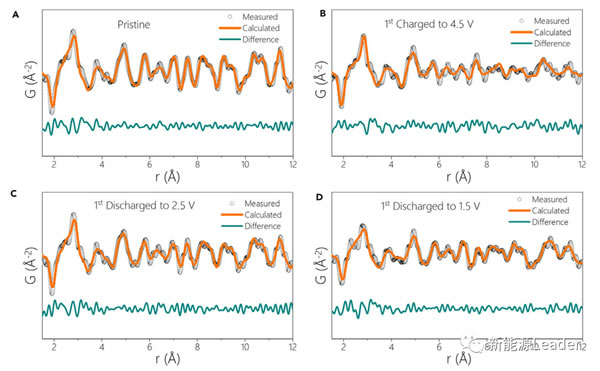
Because the neutron scattering length of element O is large, the neutron pair distribution function (nPDF) is a powerful tool for analyzing the structural changes caused by the element redox reaction. From the figure a below, we can see that it is pure for new materials. The P2 phase can achieve a very good fitting effect. However, in the charged state, only the P2 phase cannot be used to accurately fit the scattering structure. The P2 core O2 mixed phase is used, and the O vacancy factor can be considered to improve the accuracy of the fitting result, but we compare after charging and after discharging The seed scattering structure of the material can see that the two have a very large similarity, so this also indicates that although there are some miscellaneous phases in the material under the charged state, the P2 phase is still dominant.
If the Na0.72 [Li0.24Mn0.76] O2 material is discharged to 1.5V, it will inevitably cause the Mn element to participate in the reaction, resulting in the production of Mn3 + in the material, and we know that there is John-Teller effect in Mn3 + resulting in the phase change of the material (P2 is converted to P2 '), but in Na0.72 [Li0.24Mn0.76] O2 material, even if the material is discharged to 1.5V, we can still use the P2 phase to fit the material well, which shows that the The material can well suppress the John-Teller effect of Mn3 +, thereby maintaining the stability of the material structure.
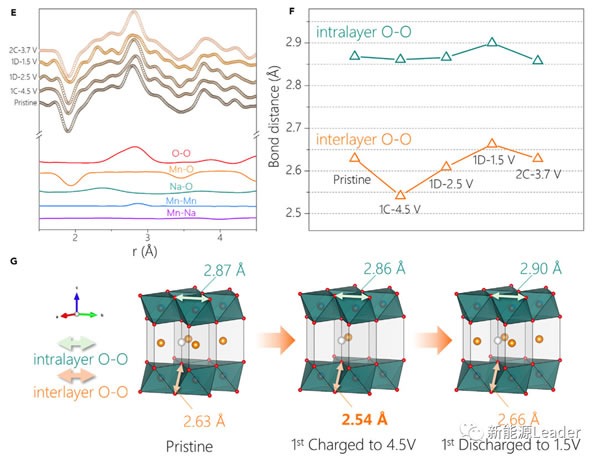
By fitting nPDF, we can also get the OO distance within and between layers (the results are shown in the figure below). From the figure we can see that the Na0.72 [Li0.24Mn0.76] O2 material is interlayer after charging The OO distance is significantly shortened, which also indicates that the O element is oxidized during the charging process, which is also strong evidence that the O element participates in the charge and discharge reaction of Na0.72 [Li0.24Mn0.76] O2 material.
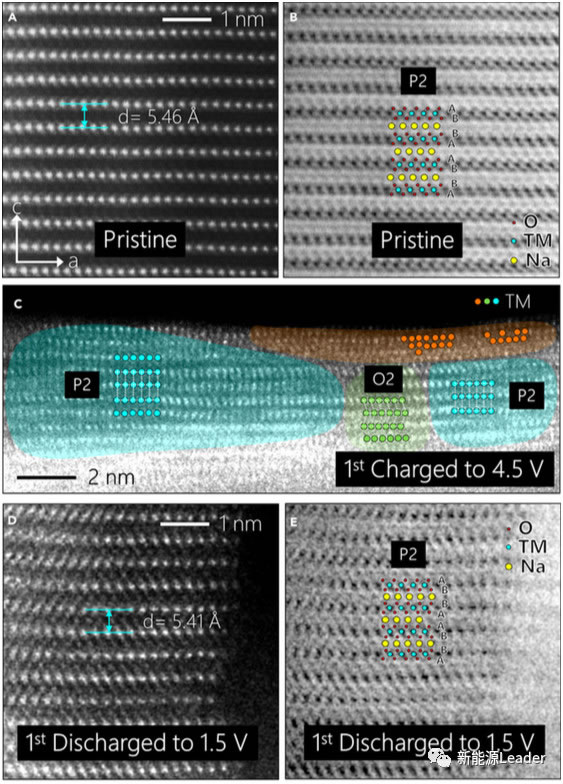
Transmission electron microscopy technology can help us observe the internal structure of the material. From the figure below, we can see that the fresh material and the material after the first discharge are both P2 layered structures. For the charged material, the surface of the material still uses the P2 structure as Mainly, but there is a small amount of O2 structure phase, accompanied by cation mixing and displacement. The generation of this cation mixing and heterophase will cause an irreversible phase change on the surface of the material, which may have a certain impact on the cycle stability of the material.
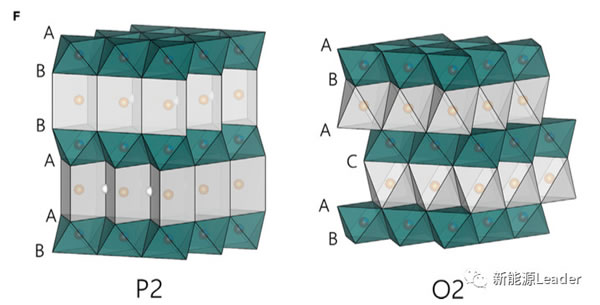
The Na0.72 [Li0.24Mn0.76] O2 material developed by Xiaohui Rong et al. Achieved a reversible capacity of 270mAh / g through the reversible redox reaction of O element. It is currently the highest reversible capacity cathode material for Na-ion batteries. The cycle performance needs to be improved, but it provides new ideas for us to develop the next generation of high-capacity cathode materials.
Gift Bags,Gift Bag Hiking Travel Pack,Eco Gift Bag,Gift String Bag
Dongguan C.Y. RedApple Industrial Limited , https://www.zjhpgbags.com